Recommended
CGD NOTES

REPORTS
Introduction
Emerging infectious diseases pose an ever-increasing threat to global health security, with population growth, urbanization, and global transit contributing to larger and more frequent outbreaks and epidemics than ever before.[1] We know the human and economic costs of these outbreaks all too well from recent experiences with Ebola, while the full toll of the 2019 novel coronavirus (COVID-19) remains to be seen.[2], [3], [4] In the face of these epidemic threats, vaccines and therapeutics are among our most important public health tools to combat and mitigate the harms of widespread infection—but only if they can be deployed at the right time and to the right people. In many cases, this will require having stockpiles at the ready to deploy vaccines where they are needed most, as well as manufacturing capacity to scale up supply to meet global demand.
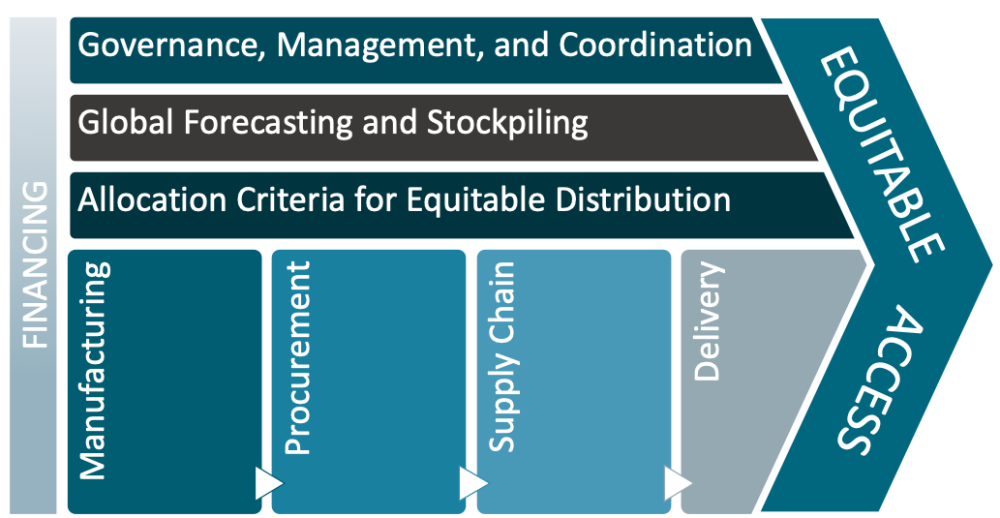
While there has been much interest and investment in developing epidemic vaccines and medicines to combat emerging infectious disease threats, there has been less attention to how we will manage and allocate the global supply of efficacious vaccines and treatments once we have them. The launch of the Access to COVID-19 Tools (ACT) Accelerator marks an unprecedented commitment to global collaboration to ensure rapid and equitable access to medical countermeasures for COVID-19, such as vaccines and treatments. Bringing together leadership from WHO, global health development partners and foundations, heads of government including from the UK and EU alongside special envoys, as well as industry partners marks a critical step to coordinate efforts around research, production, allocation and distribution of vaccines and therapeutics. Yet, to deliver on the promises of equitable access for all, there are many challenges—scientific, operational, and ethical—that must be tackled head on. Guidance is sorely needed to inform appropriate production and manufacturing, financing and procurement, supply chain, and allocation of emergency vaccine and therapeutic supply—all with attention to governance, coordination, and management of emergency supplies and stockpiles.
Background
Since the emergence of the ongoing COVID-19 crisis, billions of dollars have been mobilized to advance development of new medical countermeasures and manufacturing capacity to rapidly ramp up supply of successful candidates.[5] These new investments have built upon many mechanisms put in place following the West Africa Ebola epidemic of 2014-2015 to accelerate R&D for epidemic threats, such as the WHO R&D Blueprint[6] to coordinate global research efforts and the Coalition for Epidemic Preparedness Innovations (CEPI),[7] alongside new investments from various government research agencies, foundations, and industry partners,[8] including the recently launched COVID-19 Therapeutics Accelerator[9] and funding support for enhanced vaccine manufacturing capacity by the Gates Foundation[10] and BARDA.[11]
These investments and coordination mechanisms are critically important to ensure we will have effective medical countermeasures (MCM) to combat the emergence and reemergence of deadly pathogenic threats like COVID-19. Yet, significant questions remain about what more is needed to have adequate supply of these new vaccines and therapeutics in our global arsenal, and how they can be best deployed to combat large-scale global threats.
In the face of the current pandemic threat, the challenge of how initial and ongoing supplies of MCM for COVID-19 will be allocated across different country settings and population groups in need looms large, and requires immediate attention to help ensure the promise of “equitable access” is fulfilled. But it also presents an opportunity to put in place mechanisms and guidance for appropriate stewardship of the global supply of therapeutics and vaccines against the broader range of priority pathogens with high epidemic potential.[12] As a CEPI representative recently pointed out, “there is no agreement yet on the principles or rules for a fair allocation system incorporated into contracts that can be consistently applied and enforced. There is also no global entity responsible for ordering the manufacturing of vaccines on a global scale and paying for it.”[13] We also know from the experiences of vaccines for pandemic influenza,[14] HPV,[15] and rotavirus[16] that, without such mechanisms, limited supply will go disproportionately to high-income settings, leaving poorer nations with delayed, insufficient, or suboptimal vaccines and therapeutics supplies for those who may face even greater risk of disease and death.
A role for stockpiles and global procurement mechanisms
For many products, establishing global stockpiles seems a natural next step to ensure adequate supply for a rapid response to outbreaks. In fact, Gavi, the Vaccine Alliance recently approved a new global emergency stockpile of Ebola vaccines with an estimated investment of US$ 178 million between now and 2025 to ensure Ebola vaccines are available to combat future outbreaks.[17] And the creation of emergency stockpiles was always part of the envisioned trajectory for vaccines developed through CEPI R&D investments.[18] Global stores will be particularly important for countries lacking the resources to procure and maintain their own national stockpiles.[19] For instance, the creation of the Oral Cholera Vaccine stockpile has clearly demonstrated its value in providing coverage to poor countries like Haiti and the DRC, with over 13 million doses deployed in humanitarian crises and outbreaks in 2019 alone.[20], [21] Moreover, stockpiles may help counterbalance current market disincentives for developers and manufacturers by creating larger and more predictable demand for vaccines and therapeutics targeting emerging and re-emerging pathogens.
While we can build on the experience of existing global vaccine stockpiles, such as the WHO Smallpox Vaccine Emergency Stockpile and the Yellow Fever, Meningitis, and Cholera stockpiles currently overseen by the International Coordinating Group on Vaccine Provision, recent evaluations of the ICG have highlighted some of the current weaknesses, with calls for improved governance, more clearly defined roles of engaged parties, and greater transparency in decision-making.[22], [23] The establishment and oversight of new vaccine stockpiles will only add to the financial, operational, and ethical challenges and complexities—especially in the face of larger and more frequent outbreaks. Similar challenges and questions will arise surrounding the global supply of antiviral agents and novel therapeutics—and there is much to learn from past experiences of inefficient and inequitable use of antivirals for influenza.[24], [25]
But there are many questions that require further investigation.
With many novel vaccines and therapeutics in the pipeline—for COVID-19 and other emerging epidemic threats—how should decisions be made about which global stockpiles will be established to maintain a ready supply in the event of an outbreak? How can we appropriately forecast the demand for epidemic MCM to ensure an adequate and appropriate supply to effectively and equitably protect at-risk populations? How should clinical trials be designed to inform optimal implementation and allocation strategies? What types of institutional structures, processes, and funding mechanisms will be needed to maintain and oversee MCM supplies for different types of threats? And in the event of a large-scale or pandemic threat, like the one we now face, how will decisions be made about the equitable allocation and prioritization of epidemic vaccines and therapeutics—including which countries will have access to vaccine and therapeutic supplies as well as which population groups will be eligible given supply constraints and how provision ought to be sequenced given population and product characteristics?
Setting the research agenda for global MCM supplies
If we want to ensure that we have adequate and equitably distributed COVID-19 vaccines and therapeutics, on a global level, work is needed now to critically examine these issues and set up new norms, processes, and governance structures; financing instruments; and evidence-based approaches to inform optimal strategies. Facing these challenges head on in the context of COVID-19 can lay the groundwork for new mechanisms to better address future emerging epidemic threats and mobilize MCM where they can do the most good for those most in need. The following are key areas for research to inform new approaches for global procurement and provision of epidemic vaccines and therapeutics:
Institutional arrangements for the management and governance of global stockpiles. To inform how global institutions and coordinating groups/mechanisms should operate in this space, we need to critically examine existing structures to understand their strengths and weaknesses—and to determine which entity(ies) are best suited to host this function, or whether a new global entity is needed to oversee supplies for epidemic and pandemic threats. This includes assessing the features and technical capacities that any such entity would need to function as a trusted administrator of these critical resources, including aspects related to representation, transparency, evidence-informed policy, and oversight.
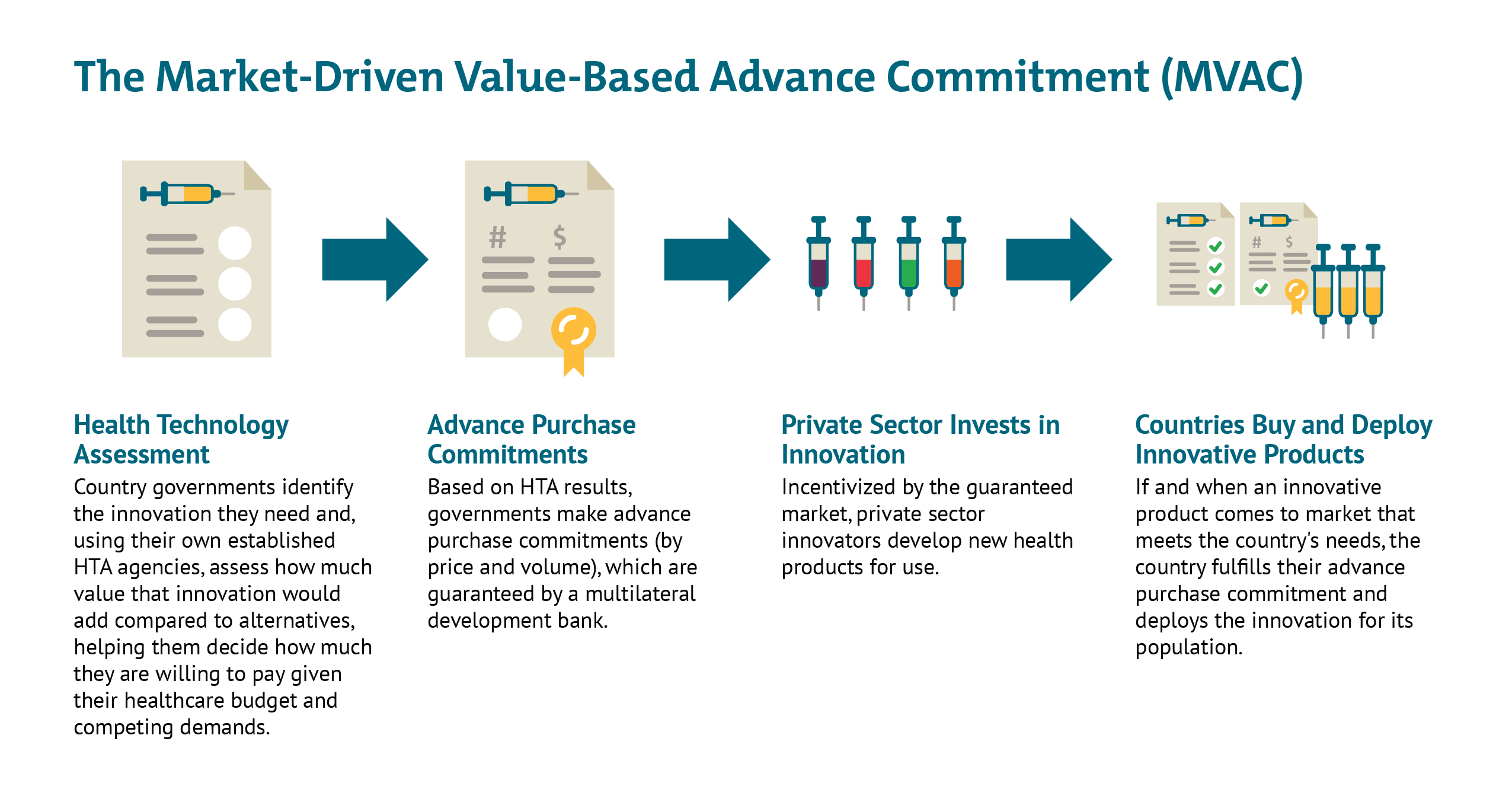
Financing of MCM. As we have discussed elsewhere, different types of epidemic vaccines and therapeutics may require innovative push and pull mechanisms to de-risk the space for industry to aggressively pursue R&D, while also providing commitments to a share of the global supply for lower- and middle-income countries that may otherwise not be able to compete with the purchasing power of larger and/or wealthier nations. Further analysis is needed to better evaluate options for using advance market commitments; market-driven, value-based advance commitment (MVAC) approaches; vaccine bonds; and other innovative financing instruments to ensure adequate and equitable supply of lifesaving prevention and treatment modalities.
Decisions to establish new stockpiles for emerging threats. Given the tremendous cost associated with establishing and maintaining stockpiles, and the range of products in the R&D pipeline, further work is needed to establish the criteria and evidence that will be used to inform decisions about new vaccine and therapeutic stockpiles, with attention to the nature of the pathogenic threat, product characteristics, manufacturing requirements, cost-effectiveness, and the appropriate volume and mix of products that will compose stockpiles for various kinds of epidemic threats.
Guidance for the allocation and distribution of vaccines and therapeutics during epidemics and pandemics. Because there will be many cases when demand exceeds supply, tough choices will have to be made about how to appropriately allocate vaccines and therapeutics from the global stockpile. Additional work is needed to explore different allocation scenarios and establish appropriate criteria, including attention to what will be most effective in reducing transmission and mortality, which populations are in greatest need, cost-effectiveness, capacity to deploy vaccines and therapeutic options in different contexts, and tradeoffs between approaches that target the most at-risk with fewer doses versus those that prioritize herd immunity of a population. This type of work could help further align and tailor biomedical research and modelling efforts to inform critical policy questions around optimal allocation strategies, within and between populations.
Combined, this new research should support the development of a stockpiling simulation model to serve as a global public good for ongoing assessment of projected need, investment, and distribution options of various MCM products to combat future epidemic threats. Developing definitive guidance and tools for these complex and contentious issues will require a broad coalition of stakeholders, bringing a range of relevant expertise to the table. This includes the collective experience and expertise of those who have experience in:
- clinical development pathways and trial design
- economics of vaccine and drug R&D
- cost-effectiveness and health technology assessment
- forecasting and infectious disease modelling
- vaccine and drug manufacturing
- legal and regulatory aspects, including international health regulations
- global health governance and institutional organizational structures
- ethics of resource allocation
- technical aspects of resource allocation
- supply chain and distribution
The global COVID-19 pandemic has called attention to the many ways in which we are still underprepared to face emergent infectious disease threats, including how to ensure biomedical interventions can get to the people who need them most and the places where they can do the most good. But this crisis also provides opportunities to forge new pathways to align evidence generation, ethics analysis, economics, and institutional governance in service of the promise of equitable access to vaccines and therapeutics, for this epidemic and the next inevitable threat. The launch of the ACT Accelerator already brings together various heads of government and health leaders from WHO, CEPI, Gavi, the Global Fund, UNITAID, the Wellcome Trust, the Bill and Melinda Gates Foundation, the International Red Cross and Red Crescent Movement (IFRC), the International Federation of Pharmaceutical Manufacturers (IFPMA), the Developing Countries Vaccine Manufacturers’ Network (DCVMN), and the International Generic and Biosimilar Medicines Association (IGBA), alongside other key partners. This initiative presents an unprecedented moment for global leadership to invest in much-needed research to overcome the scientific, operational and ethical complexities and challenges on the path to equitable access to epidemic vaccines and treatments.
[1] Bloom DE, Black S, Rappuoli R. Emerging infectious diseases: A proactive approach. Vol. 114, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences; 2017. p. 4055–9.
[2] CDC. [Internet] Cost of the Ebola Epidemic [In https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/cost-of-ebola.html]
[3] World Economic Forum [Internet]. The human costs of epidemics are going down but the economic costs are going up. Here’s why. [cited 2019 Nov 26]. Available from: https://www.weforum.org/agenda/2018/05/how-epidemics-infect-the-global-economy-and-what-to-do-about-it/
[4] Davies R. How coronavirus is affecting the global economy. The Guardian. 5 February 2020. Available from: https://www.theguardian.com/world/2020/feb/05/coronavirus-global-economy
[5] WHO Coronavirus disease (COVID-2019) R&D. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/ [accessed 10 April 2020]
[6] World Health Organization (WHO). An R&D Blueprint for Action to Prevent Epidemic - Plan of Action May 2016. 2016.
[7] CEPI [Internet]. Our Portfolio. Available from: https://cepi.net/research_dev/our-portfolio/ [accessed 10 April 2020)
[8] COVID-19 R&D tracker. https://www.ghtcoalition.org/resources-item/covid-19-r-d-tracker [accessed 10 April 2020)
[9] https://www.gatesfoundation.org/Media-Center/Press-Releases/2020/03/COVID-19-Therapeutics-Accelerator
[10] https://www.businessinsider.com/bill-gates-factories-7-different-vaccines-to-fight-coronavirus-2020-4
[11] https://www.hhs.gov/about/news/2020/03/30/hhs-accelerates-clinical-trials-prepares-manufacturing-covid-19-vaccines.html
[12] https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts
[14] Fidler DP (2010) Negotiating Equitable Access to Influenza Vaccines: Global Health Diplomacy and the Controversies Surrounding Avian Influenza H5N1 and Pandemic Influenza H1N1. PLoS Med 7(5): e1000247. https://doi.org/10.1371/journal.pmed.1000247
[15]https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/WHO_HPV_market_study_public_summary_Dec2019.pdf
[16] https://www.npr.org/sections/goatsandsoda/2018/11/01/655844287/merck-pulls-out-of-agreement-to-supply-life-saving-vaccine-to-millions-of-kids
[18] Røttingen JA, Gouglas D, Feinberg M, Plotkin S, Raghavan KV, Witty A, Draghia-Akli R, Stoffels P, Piot P. New vaccines against epidemic infectious diseases. N Engl J Med. 2017 Jan 18.
[19] Yen C, Hyde TB, Costa AJ, Fernandez K, Tam JS, Hugonnet S, et al. The development of global vaccine stockpiles. Vol. 15, The Lancet Infectious Diseases. Lancet Publishing Group; 2015. p. 340–7
[20] Desai SN, Pezzoli L, Alberti KP, Martin S, Costa A, Perea W, Legros D. Achievements and challenges for the use of killed oral cholera vaccines in the global stockpile era. Human Vaccines & Immunotherapeutics, 13:3, 579-587, DOI: 10.1080/21645515.2016.1245250
[21] Dashboard: International Coordinating Group (ICG) on Vaccine Provision on cholera [Internet]. Available from: https://www.who.int/csr/disease/icg/cholera-dashboard/en/ (accessed 5 February 2020)
[22] External Evaluation of the International Coordinating Group on Vaccine Provision (ICG) Mechanism. October 2017. Available from: https://www.who.int/docs/default-source/documents/evaluation/external-evaluation-vaccine-group.pdf?sfvrsn=c197d7e4_2
[23] Review of the International Coordinating Group on Vaccine Provision (2006-2016) [Internet]. 2016 [cited 2019 Nov 26]. Available from: http://apps.who.int/bookorders.
[25] Carrasco LR, Lee VJ, Chen MI, Matchar DB, Thompson JP, Cook AR. Strategies for antiviral stockpiling for future influenza pandemics: a global epidemic-economic perspective. J R Soc Interface. 2011;8(62):1307–1313. doi:10.1098/rsif.2010.0715
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.