Recommended
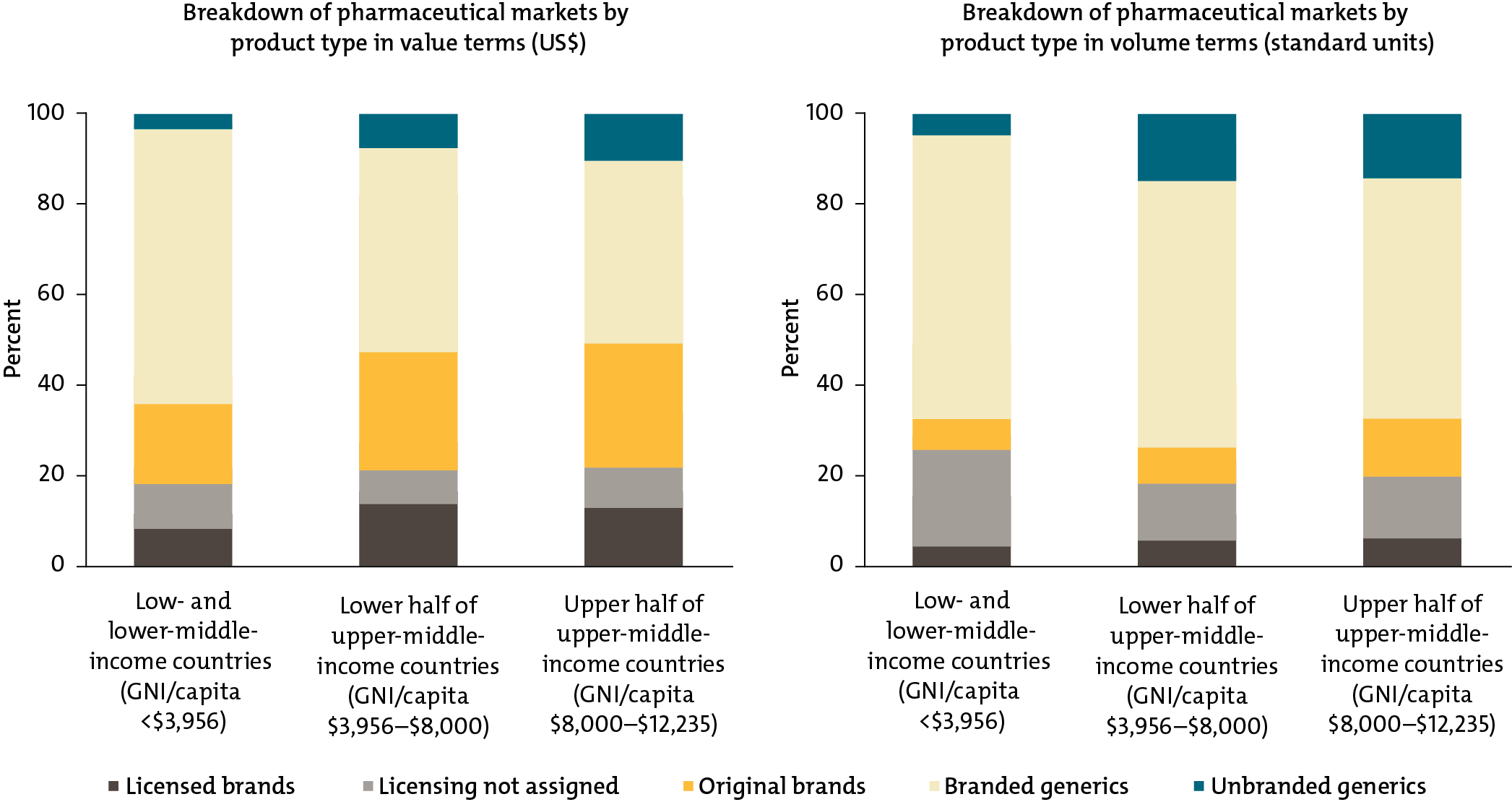
Low- and middle-income country governments and global health funders are already spending big money on health products like medicines, diagnostics, and devices. In just 43 of these countries, our research found that spending on health products totals $50 billion per year. To achieve universal health coverage and provide their citizens with access to quality and affordable medicines, countries will need to buy these lifesaving products efficiently and effectively.
But a triple transition is on the horizon and global health procurement needs are evolving rapidly. Countries are transitioning from donor aid, global disease burdens are shifting from infectious to non-communicable conditions, and health systems are advancing from disease-specific programs to universal health coverage. How can the global health community act now to better prepare for these sweeping changes, to ensure the efficiency, quality, affordability, and security of global health procurement? CGD’s Working Group on the Future of Global Health Procurement has some answers.
In our final report, released today, the Working Group considers how global health procurement bodies can reimagine and adapt their roles to stay relevant in a changing world.
1. Sustain and expand global cooperation for procurement and targeted innovation
If we want to sustain and expand global access to quality, affordable health products, the end of traditional health assistance—so-called aid transition—cannot be the end of global health cooperation for effective procurement. Instead, global health entities must redefine their procurement support to adapt to the changing context. For example, global or regional pooling could help address fragmented demand, especially in low-volume or fragile product markets; donors can also partner with middle-income-country payers to identify and advance local R&D priorities. Donors could even consider continued post-transition subsidy for specific health products—for example, products with important global benefits or which are marginally cost-effective.
2. Reform WHO guidance and policy to support modern and agile procurement policy and practice
Many LMICs look to the WHO for guidance on procurement and pharmaceutical policy. But in many cases WHO guidance in these areas is out of date, inflexible, and not adapted to local contexts. Going forward, the WHO should work with countries to help adapt the WHO essential medicines, diagnostics, and medical devices lists and technical guidance to local contexts and resource constraints. Building on the Collaborative Registration Procedure, the WHO should also expand efforts to facilitate expedited drug registration at the country level to enhance healthy competition in generic markets.
3. Professionalize procurement by building capacity and driving strategic practice
Until procurement is professionalized as a core health system function, inefficiencies in procurement mechanisms will continue to jeopardize global health progress. A network of country procurement bodies, global procurement agents, and multilateral institutions could help professionalize procurement by creating standardized performance measures, global health-specific procurement resources, mentorship and exchange programs, and Procurement University—an intensive short course on best practices for procurement officials.
4. Support in-country procurement policy reform
It’s time for country governments to address the institutional and legal barriers that lead to suboptimal procurement outcomes—for example, onerous product registration processes, local purchasing requirements that limit competition, and legal or regulatory strictures against more effective tendering and contracting modalities. Governments themselves will need to take the lead on a reform agenda, but global funders can and should play and important supporting role. For example, development policy lending from the World Bank could support a government-led agenda for policy and institutional change.
The Working Group understands that implementing these recommendations will be difficult, spanning many years and requiring the buy-in of diverse actors. But by taking these steps, the global health community can dramatically expand access to lifesaving health products for the world’s neediest. It’s time to act.
To read the final report of CGD’s Working Group on the Future of Global Health Procurement, visit cgdev.org/better-health-procurement.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.