Recommended
Diagnostic testing is at the center of the policy debate around COVID-19 interventions in India. As of June 1, 2020, India had conducted approximately 3.8 million tests since it began testing in February,[1] but many experts have noted that testing capacity is still drastically insufficient for the needs of the population. Daily COVID-19 tests per 1,000 people are only 0.08 in India compared with 1.16 in the United States and 1.02 in Italy (as of May 30, 2020). India’s COVID-19 test positivity rate is about 5 percent (as of June 1, 2020)—within the World Health Organization’s recommended rate of <10 percent. While this number might suggest that testing capacity is currently sufficient for the size of the outbreak,, analyses indicates that a nearly tenfold increase in testing is required to keep the positivity rates (reassuringly) low. Delays in testing have hampered the COVID-19 response in Europe, the United States, and Brazil; inadequate testing capacity in India may prove to be an even greater challenge.
Figure 1. Daily COVID-19 tests per 1,000 people
Source: Our World in Data.
The Indian government’s current approach to testing has three key aspects:
- The Indian Council for Medical Research (ICMR) recommends real-time RT-PCR[2] throat/nasal swab tests as the gold standard for COVID-19 diagnosis.
- The Indian government recommends pooled sample screening by PCR in areas and for populations with COVID-19 positivity rates of <5 percent to increase laboratory capacity for screening surveillance samples.
- Authorities are simultaneously looking for a reliable rapid antibody test for epidemiological and surveillance purposes.
The RT-PCR throat/nasal swab test has been the key diagnostic test used in India. The rapid antibody tests were withdrawn by ICMR within a few weeks of use over reliability concerns. However, ICMR has recently encouraged states to conduct serosurveys using an IgG ELISA[3] test. While the RT-PCR test diagnoses current infection by detecting the presence of live virus, the antibody test provides information about the history of infection by detecting antibodies specific to COVID-19. In the early stages of an epidemic, or after a period of suppression, RT-PCR is useful to identify infected persons, trace their contacts, and isolate them to limit the spread of infection. The rapid antibody tests are better suited to epidemiological studies and surveillance assessments of the proportion of a population with immunity to COVID-19. Such tests can help guide social isolation policies and potentially get people who are immune back to work. The differences between the two tests are summarized in appendix 1.
There are currently over 100,000 tests being carried out in India every day (e.g., 100,180 tests were conducted on June 1, 2020). The government aims to double this number—to 200,000 tests—but as the experiences of Europe and the United States show, testing would actually have to expand tenfold or more over the next few weeks to accurately estimate the true number of cases, to achieve a meaningful epidemiologic understanding of the prevalence, and to guide measures as the economy reopens. Such a large-scale increase of COVID-19 testing in India would put the testing supply chain’s scaling-up ability to the test. There have already been a number of recent reports highlighting shortages in essential supplies for COVID-19 testing, long turnaround times for test results, and delays in timely notification and isolation of positive cases. Testing has value only when there is proper interpretation of test results and mandated follow-up actions related to containment. In this note, we explain how the SARS-CoV-2 testing supply chain in India is organized, describe the underlying structural bottlenecks, and provide some recommendations for how the country can expand timely and effective testing for the battle against COVID-19.
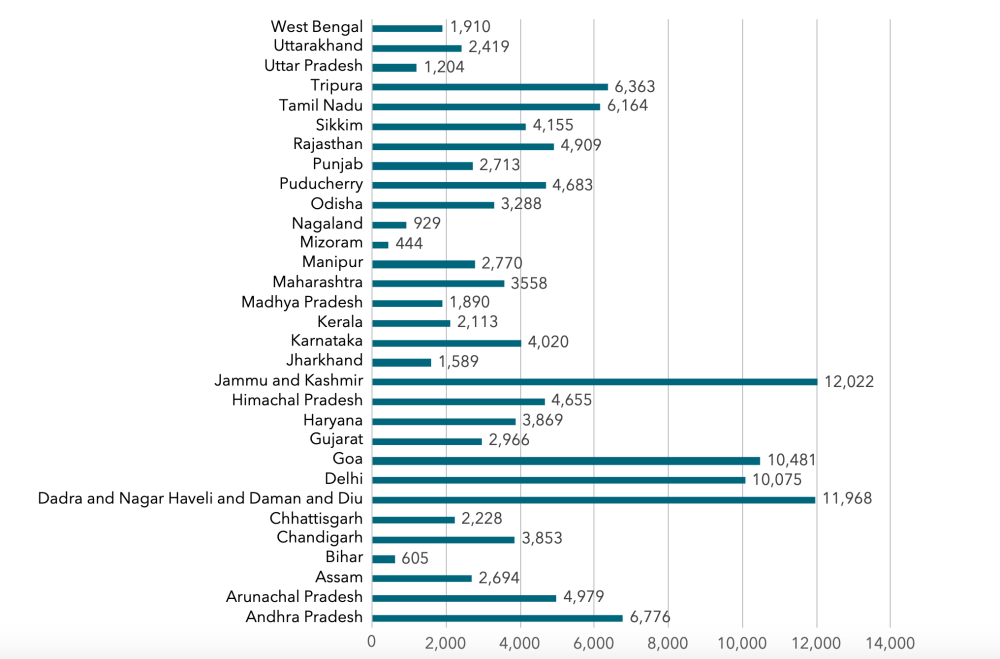
Health is a state subject in India. While the central government designs the testing strategy, the states implement it. This has resulted in variations among states in the scale of testing. Overall preparedness and response to COVID-19 have also differed from state to state.
Figure 2. Tests conducted per million people, by state (as of May 29, 2020)
Source: Authors’ own calculations based on data from https://www.covid19india.org.
Test sites and laboratories: evolving capacities and standard operating procedures
ICMR is the lead agency for the COVID-19 testing strategy in India. It began the COVID-19 testing operation by leveraging the approximately 106 Viral Research and Diagnostic Laboratories (VRDL) it had helped establish over the last few years at the regional, state, and medical-college level. Initially, the VRDLs were the only sites designated to collect samples for COVID-19, which they would then send to the National Institute of Virology (NIV) in Pune for RT-PCR testing. A contracted courier agency transported the specimens from the VRDLs to the central NIV lab. Over time, government operating procedures changed to allow the VRDLs to carry out the tests themselves. Subsequently, testing was initiated in partnership with laboratories in the Department of Science and Technology, Department of Biotechnology, the Indian Council of Agricultural Research, the Council of Scientific and Industrial Research, the Defense Research and Development Organization, the Ministry of Human Resource Development, medical colleges, and private laboratories. As of May 31, there were 472 government-owned labs and 204 private laboratories authorized for lab testing.
There are three types of molecular-based tests currently being used: ICMR-validated RT-PCR , MolBio Diagnostics’ Truenat, and Cepheid’s cartridge-based GeneXpert. A breakdown (as of May 31, 2020) of these tests, which are being used nationwide in the 676 public and private laboratories testing for COVID-19, and which are reporting to ICMR are:
- Real-time RT-PCR for COVID-19: 481 (government: 313 + private: 168)
- Truenat test for COVID-19: 140 (government: 131 + private: 9)
- CBNAAT[4] for COVID-19: 55 (government: 28 + private: 27)
When a VRDL first begins conducting testing, it must send its first 10 negative samples to NIV for cross-validation. It must also send a proportion of the all positive test samples to NIV (private labs must send all positive samples). Blood and serum samples must be collected from all positive patients and sent to NIV. This creates additional sample flows in the testing supply chain, which are presently being met but that could become a challenge if capacity (in terms of number of labs and/or of number of tests conducted per lab) increases tenfold. Further, given that a cold chain must be maintained throughout sample transit, such significant additional sample flows would increase the risk of cold-chain breaches.
The Indian government has been continuously attempting to adapt and update standard operating procedures to improve its testing abilities. In addition to making testing labs more available, it has revised its initial recommendation that Truenat only be used as a screening test requiring confirmation by a RT-PCR test. ICMR now declares the Truenat system to be a comprehensive assay for screening and confirmation of COVID-19 cases. While RT-PCR is limited to well-equipped hospitals, Truenat can be deployed at district hospitals across the country.
Leveraging the country’s private sector: progress in made-in-india supplies, but more is needed
The Indian government has partnered with domestic industry to build self-reliance in its testing capacity. The indigenous production of swabs for testing COVID-19 has begun, with more than 200,000 swabs now being manufactured per day. Fourteen of the 28 RT-PCR testing kits approved by ICMR validation centers are locally produced. An indigenous manufacturer has also developed a viral extraction kit. Truenat, a fully indigenous diagnostic platform developed for tuberculosis, has now been validated by ICMR for COVID-19 testing. And a completely indigenous IgG ELISA test for antibody detection of SARS-CoV-2 has been developed and validated by NIV. There is now a greater openness toward various government research and development labs to collaborate with private industry in production.
As mentioned earlier, to augment the Indian government’s testing efforts, 204 private labs are also testing. This number is low because COVID-19 testing in private laboratories is limited to those with an accreditation certificate from the National Accreditation Board for Testing and Calibration Laboratories (NABL), and the scope of accreditation includes real-time PCR for RNA. There are only 223 private labs out of more than 1,100 NABL labs in India that meet these criteria (government laboratories are exempt from such stringent rules), limiting the ability to substantially harness the country’s private sector testing capabilities. Adding to the regulatory obstacles, private labs have stressed that the procurement of specialized and expensive equipment, trained manpower, and space are major barriers to initiating COVID-19 testing. Substantial capital investment is needed for every new COVID-19 testing facility.. Private labs (but not government labs) are also required to transport all positive samples to NIV leading to costly and time-consuming sample flows. Finally, private labs may be further discouraged by the stigma associated with COVID testing, resulting in a substantial decrease in routine lab work.
Procurement and distribution
The primers, probes, master mix, positive control, and RNase P[5] that government labs require are procured by the central government of India (until recently by ICMR and now by HITES[6]). At the very start of testing, supplies were being shipped to the testing labs from the stocking depots and storage locations at NIV Pune or ICMR Delhi. Originally, orders placed to suppliers were received at these two sites, but over the past few weeks another 14 regional depots have been introduced for supply distribution to expedite the process. Appendix 2 lists the regional depots for the storage and transportation of COVID kits. These 16 regional depots (including two central depots) have been assigned government laboratories for distribution of COVID-testing-related supplies. The intent was to remove bottlenecks from the system by decentralizing stockholding closer to the testing labs. The labs can now order their supplies directly from these depots, and the depots must forecast and communicate their needs for the coming weeks to ICMR/NIV, which then communicates with suppliers for direct delivery to the depot.
In addition to supplies provided by ICMR, state governments are responsible for suppling viral transport media for sample collection, RNA extraction kits, and other needed consumables. While ICMR is responsible for supplies related to the step of conducting RT-PCR, the states procure supplies related to the sample collection and RNA steps. Variations among states in their adopted procurement mechanisms are likely. The regional depots may be processing both lab-level and state-level stock requests and deciding quantity and items to be dispatched to each linked lab and state. Managing the distribution of supplies related to COVID-19 testing but procured through different mechanisms adds complexity for these depots. Appendix 3 provides details on the assignment of procurement responsibilities between ICMR and state governments.
Private laboratories are required to procure their own supplies for carrying out COVID-19 tests. The government had previously capped the price of COVID-19 tests by private labs to a maximum of INR 4,500. Recently, ICMR recommended that state governments and union territory administrations negotiate with the private sector to arrive at lower prices because there has been a decrease in testing-related costs due to increased market competition and availability of indigenous testing materials and kits, which has driven down testing supply costs.
In addition to PCR testing, ICMR had also procured antibody-based rapid diagnostic tests (such as ELISAs), which pick up past (as opposed to active) infection. These tests were withdrawn within a few weeks of their introduction but have recently been permitted again.
Recommendations for action
1. Build a scientifically rigorous but pragmatic testing strategy
An important element of a scaled-up testing strategy is how to use a combination of PCR tests, antibody tests, and at-home testing. While the accuracy, sensitivity, and overall quality of antibody tests has been difficult to ascertain, many countries, including the United States and the United Kingdom, have now started using antibody tests. High-quality and reliable antibody tests will be crucial to identifying pockets of infection at a large scale and to identifying those with immunity. They will enable the central and state governments to become more targeted and precise about the geography and nature of future lockdowns in the future. ICMR has recently recommended that states conduct serosurveys using an IgG ELISA test, which it has developed and shared with many Indian manufacturers (see ICMR tweet here). This is a good step toward including the use of antibody testing as part of an overall testing strategy.
The pooling of RT-PCR samples is a cost-effective technique for testing that is evidence-based and can help ramp up testing with the resources available. This strategy has been useful for surveillance of migrant workers and international passengers in institutional quarantine.
At-home testing and specimen collection is likely to become a more widely used testing method in the future. The US Food and Drug Administration has authorized at-home tests (i.e., self-collection of samples) from at least five companies and home collection of saliva samples rather than nasopharyngeal swabs. India’s private lab network has been a global leader in terms of the extensive reach of its at-home sample collection capabilities. Over time, plans should scale-up safe (e.g., with adequate PPE[7] and safety protocols) at-home sample collection for COVID tests.
Testing for COVID-19 is now available for free through private labs to 500 million people under Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB-PM JAY). The National Health Authority have advised state health agencies to empanel ICMR-approved labs, negotiate prices for testing, and help empaneled hospitals connect with these private labs. Tests under AB-PM JAY are to be done according to ICMR and state guidelines.
2. Ensure faster turnaround time for COVID-19 testing to inform an effective and accountable policy response
Rapid turnaround time (TAT) is desirable from multiple standpoints. Faster case detection allows for quicker isolation of confirmed cases and contact tracing, which can help to slow the spread of the disease. For patients in a hospitals and health facilities, it helps conserve PPE and reduce nosocomial infection; and for the overall response strategy, it provides more up-to-date and reliable data.
Improving TAT requires a thorough process mapping exercise to understand the value created by every step of the process and allow for a more careful analysis of the trade-offs involved. For example, private labs are required to send all positive samples to NIV. Instead, it may be more efficient to do external quality assessment using interlaboratory comparison/proficiency testing (recommended by NABL) with random sampling to decrease the burden of all samples being transported to NIV for quality control.
Similar to daily reporting of the number of tests carried out, TAT should be used as a key performance indicator and publicly reported. Routine reporting of TAT would enable faster detection of testing bottlenecks and enable their proactive resolution. It would also create greater public accountability for the Indian government’s overall COVID-19 testing strategy.
3. Design the supply chain network to withstand the anticipated short- to mid-term tenfold scale-up
For the testing supply chain to withstand a large-scale increase (e.g., by tenfold as we propose here), it is important to determine the optimal product sourcing and inventory deployment rules to meet the ~10X larger flows through the network. Proactively understanding which network nodes that will become constraints can help in reconfiguring parts of the supply chain before they slow down the whole system.
Not only is the demand for testing changing dynamically, testing algorithms and criteria will also have to continually adapt to new testing technology and protocols. The supply system can be “pressure tested” against demand scenarios built on different case projections, testing protocols, and projected availability of all testing equipment. Commercial companies, public sector organizations, and the military routinely carry out such supply chain network design studies with different scenarios of demand and supply capacity; a similar simulation must be run to prepare the system for effective scale-up.
Real-time visibility of product flow and inventory at different points in the system will become much more important in such a system as testing volumes continue to increase. Constraints can occur across any of the multiple components needed to conduct a test (viral transport media, RNA extraction kits, primers, probes, master mix, or combined RT-PCR test kits; see table 1 above), and a dashboard should include visibility on each of those components, along with their availability at each of the individual supply depots, and ideally at individual labs. The central government should put in place a supply chain visibility solution that can provide real-time visibility of inventory and product flows under the current system and with built-on capabilities for handling a more decentralized delivery system in the future, including in the post-COVID era as AB-PM JAY expands the use of private labs across the country.
4. Leverage the country’s private sector for labs, supplies, and logistics with a national strategy
The long-term testing strategy for COVID-19 must be based on the network of public and accredited private labs sourcing testing consumables from suppliers, whose quality has been vetted by the central government and with whom the central government has negotiated and agreed on rate contracts. The ordering and delivery of product from manufacturers to labs could then be managed by private logistics firms. India has a vast and well-functioning private sector with excellent supply chain know-how on sourcing and distribution. Organizing the testing supply chain to fully leverage such capabilities is essential.
For full appendices, please follow the link here.
[1] Source: Indian Council for Medical Research.
[2] RT-PCR stands for reverse transcription polymerase chain reaction.
[3] ELISA stands for enzyme-linked immunosorbent assay.
[4] CBNAAT = cartridge-based nucleic acid amplification test.
[5] RNase P stands for ribonuclease P.
[6] HITES stand for HLL Infratech Services Limited.
[7] PPE stands for personal protective equipment.
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.