Recommended

Blog Post
The politics of antimicrobial resistance (AMR) has changed substantially since we launched the first CGD working group on AMR in 2007. There is now much more awareness about the need to act, even if global solutions have not yet been implemented. Countries including the UK, Canada, and Japan have announced innovative new ways of purchasing antibiotics to limit overuse and generate investment needed to fund new drugs. And while the almost $200 million a year of dedicated push funding specifically for antimicrobial research and development (R&D) dwarfs the funding available a decade ago, it is still only about half of what is needed.
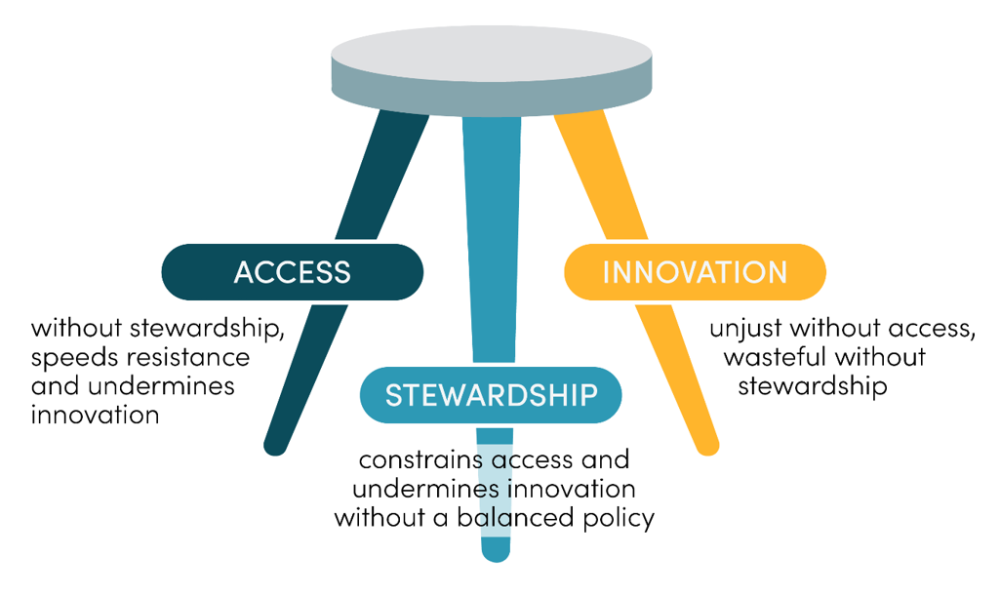
Despite these advances, the antimicrobial market is still grappling with three interwoven challenges: innovation, access, and stewardship. Innovation is waning at an alarming rate: across 25 years, there was an 81 percent drop in the number of new antibiotics approved by the US Food and Drug Administration. Inadequate access to existing and new antimicrobials is a leading cause of death from AMR infections, particularly in low- and middle-income countries (LMICs). And the way that antimicrobials are currently purchased contributes to overuse and inappropriate use: studies in Kenya, India, and the United States have found that as many as half the antibiotics prescribed in primary care are inappropriate. Stewardship that can reduce this type of unnecessary use will be in everyone’s interest. These three problems all need to be fixed to build a market that is fit for purpose.
Adapted from: Hoffman and Outterson 2015
Progress against these problems has been slow. There is no clear agreement on the optimal roles for different stakeholders or on what it looks like to be a good global citizen on preventing resistance. In much of the world, there is also a lack of evidence on best practice. This is the problem that CGD’s current working group on AMR has sought to identify solutions for.
Our work took a three-pronged approach. Firstly, we convened a working group comprising 22 experts from across the world to elicit a broad range of perspectives. These experts hailed from 12 countries including Brazil, India, Nigeria, Thailand, the United Kingdom, and the United States, and included representatives from the pharmaceutical industry, government officials, heads of global health institutions, funders, and healthcare providers from LMICs. Secondly, we conducted a landscape review to understand the current thinking on policies to tackle AMR. A key finding from this review was that there was a wide range of priorities and objectives—which could hinder collaborative action—despite the agreement on the need for global action. Thirdly, we carried out three country case studies in India, Brazil, and Kenya, as well as other research pieces to fill evidence gaps. These country case studies, led by local partners, allowed us to understand the specific local contexts in these regions and identify political barriers to action. The additional research pieces helped clarify the nature of the AMR problem and provide insight on best practice to overcome these issues.
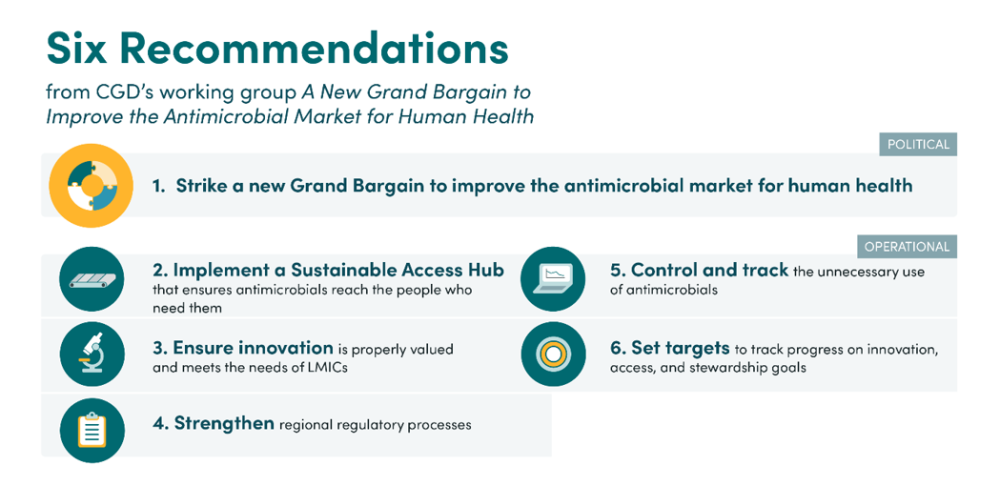
Building on this work, we are today publishing the working group’s final report, which proposes six key recommendations to improve the antimicrobial market for human health. This report will be formally launched in collaboration with the Nigerian and UK governments on the sidelines of the United Nations General Assembly, today.
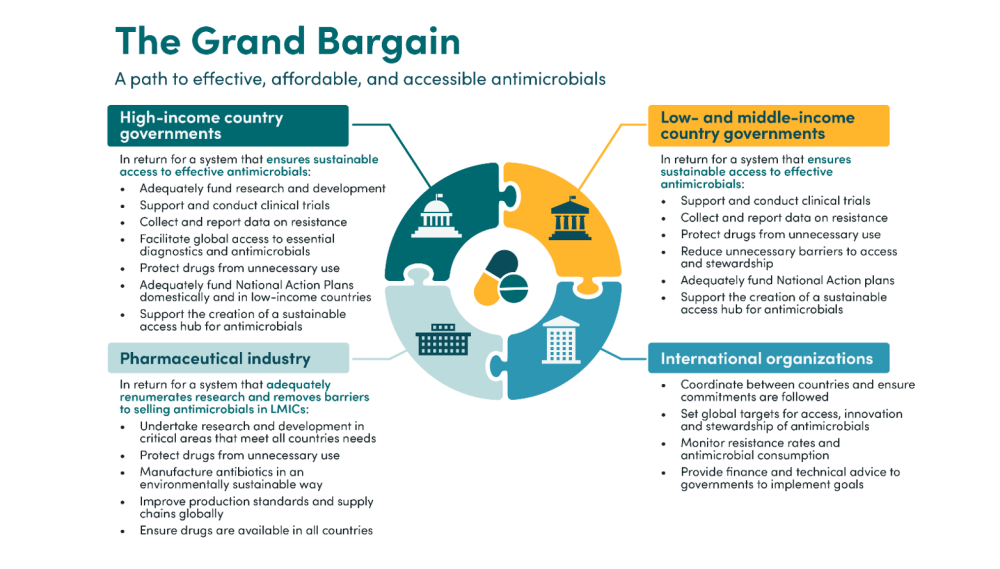
Firstly, we propose an overarching political recommendation, a “Grand Bargain” between high-income countries (HICs), LMICs, and the pharmaceutical industry. This would provide a strong set of shared principles for innovation, access, and stewardship. The political agreement would lay out the rights and responsibilities of all key stakeholders which are needed to achieve coordinated and effective global action. All countries would be expected to support clinical trials, collect, and report resistance data, protect drugs from unnecessary use, and implement their National Action Plans on AMR. HICs would additionally be expected to adequately fund research and development for new treatments, facilitate global access of these treatments, and both fund their own National Action Plans and support low-income countries with theirs. The pharmaceutical industry would be expected to undertake R&D of drugs meeting the needs of all countries, ensure global access to these drugs, and manufacture drugs in an environmentally stable way. International organisations would also have a key role to play in facilitating and supporting the implementation of the agreement. Based on engagement with key stakeholders, we believe that a mutually agreeable deal is achievable and workable—but, given the interrelatedness of problems, will require action from all stakeholder groups to be successful and sustainable.
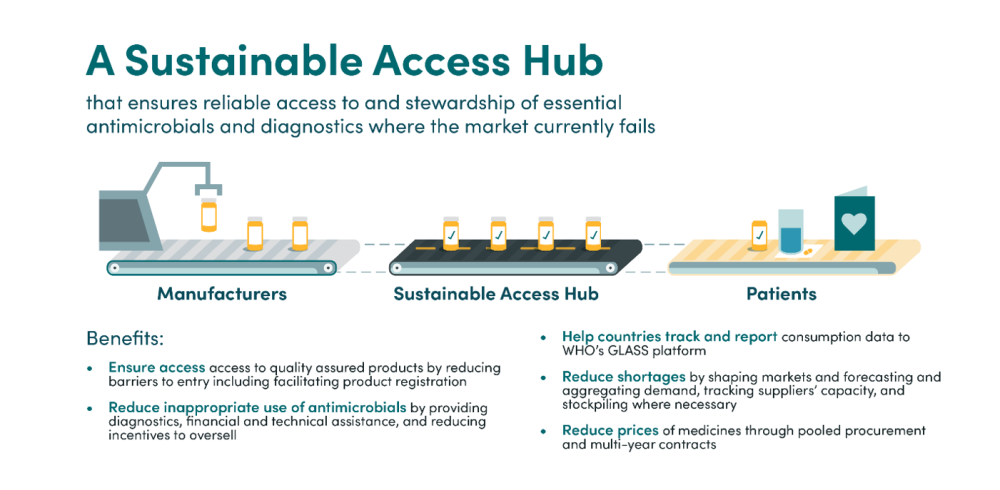
We then propose a set of operational recommendations which suggest ways to implement such a Grand Bargain. The most important of these is the implementation of a sustainable access hub (or several smaller hubs at the regional level) to address access challenges, primarily in LMICs. This would serve as a backstop to ensure reliable access to essential antimicrobials and diagnostics where the market currently fails to do so. The hub would achieve this by procuring or supporting the procurement of these products —assisting countries to determine and aggregate demand and establishing agreements with manufacturers—reducing market entry barriers by simplifying registration and distribution procedures, and stockpiling drugs to manage supply or demand shocks. Crucially, the hub would work to ensure that access problems are addressed through the lens of appropriate stewardship: the hub would track consumption data and support countries to implement surveillance systems, limit unnecessary use where high usage rates are identified, and work with procurers to reduce incentives for overselling. The hub could also provide technical and financial support to help countries adopt best practices with regards to access and stewardship. We recommend that this hub be housed at an existing institution, funded by donor governments, but with all governments playing a role in the design to ensure that it meets global needs.
Alongside these two main recommendations, we make several other operational recommendations. Countries should implement policies to ensure that innovation is appropriately valued and meets the needs of all countries, for example by updating health technology assessments to better capture the societal value of antimicrobials. International organisations should assist by updating target product profile lists to ensure innovation of products meets global needs. National and regional regulatory authorities should develop robust policies to regulate and streamline antimicrobial clinical trials, expedite market authorisation, and improve post-marketing surveillance. Governments should introduce robust systems to control and track antimicrobial use, including imposing limits on how, where, and on what evidence different antimicrobials can be dispensed. Finally, governments should set targets to track progress against innovation, access, and stewardship goals. These targets should be designed carefully so that they reflect countries’ differing resources and encourage virtuous behaviour.
These recommendations would dramatically improve the antimicrobial market for human health. While the political and operational recommendations work best in tandem, neither relies on the other. We hope that a global agreement, reflecting the Grand Bargain, can be reached when AMR returns to the UN General Assembly agenda in 2024—but the world should not wait for a global agreement to start implementing the operational recommendations. The window of opportunity to act is now if we want to avoid a global health crisis of pandemic proportions.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.