Recommended
CGD NOTES
The COVID-19 pandemic has pushed global vaccine manufacturing capacity to new limits and primed the pump for further expansion in regions like Latin America and the Caribbean (LAC) to prepare for future pandemic risks. The region has mainly relied on imports, with only 15 percent of total COVID-19 vaccine supply produced locally, which has made LAC vulnerable to nationalism, export bans, and shortages. As a result, policy makers are now interested in reducing dependance and expanding emergency vaccine manufacturing capacity to mitigate outbreaks and prevent and respond to future pandemics quickly and efficiently.
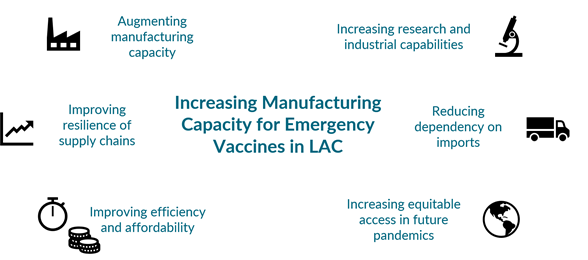
Several LAC countries—including Argentina, Brazil, Chile, and Colombia—have pledged to introduce and/or ramp up manufacturing of emergency vaccines, and regional platforms such as the Forum for the Progress and Development of South America (PROSUR) and the Pan American Health Organization (PAHO) have launched initiatives to boost vaccine production and ensure the region is ready to respond swiftly to the next pandemic threat. As regional and national policymakers translate commitments to realities, they must balance multiple, sometimes competing policy goals (see Figure 1) and address unintended consequences, identifying trade-offs and potential mitigation strategies to improve efficiency, affordability, and access in the rollout of emergency vaccines while avoiding inadequate, fragmented, and reactive approaches.
In this blog, we share a summary from the CGD note published today on the first-order demand and supply-side considerations for expanding emergency vaccine manufacturing capacity in LAC. The note expands on the factors LAC policymakers must understand and implement to increase the likelihood of successful, fit-for-purpose manufacturing initiatives that can lead to rapid and equitable access to emergency vaccines now and into the future—a critical component of broader, comprehensive pandemic preparedness policy.
Key Elements for Success on the Demand Side
These five key demand-side actions will support LAC policymakers to scale up vaccine manufacturing capacity effectively and sustainably:
-
Assuring fiscal space to spend on routine vaccine procurement as well as “ever-warm” and surge vaccine manufacturing and purchasing
The COVID-19 pandemic has caused several fiscal challenges—including a 6.8 percent contraction in gross domestic product in 2020 in the LAC region—that threaten the level of investment in developing manufacturing capacity now and procuring vaccine in the event of a pandemic threat. The uncertainty introduced by the pandemic also risks undermining adequate investment in vaccine manufacturing and purchasing, including “ever-warm manufacturing capacities” ready to scale up when pandemic-potential pathogens strike. Amidst competing, costly priorities within and outside of the health budget, such as routine immunization, governments must ensure sufficient, sustainable financing is available to produce and procure emergency vaccine.
-
Projecting future vaccine demand
The volume and timing of vaccine demand is difficult to estimate, especially for unknown future pandemic threats. To mitigate the uncertainty, governments can help ensure stable demand going forward by treating the COVID-19 and seasonal influenza vaccines as routine vaccines and establishing an ecosystem for sustainable, flexible manufacturing by manufacturing other products with stable demand projections at the newly developed sites. Governments can also leverage reasonable demand estimates that can be made for key threats like influenza and Zika virus and as well as the work of World Health Organization on the Research & Development Blueprint for Action to Prevent Epidemics to identify potential threats and anticipate manufacturing demands.
-
Assessing relation to existing procurement arrangements, such as the PAHO Revolving Fund
Scaling up domestic or regional vaccine manufacturing may lead more countries to “buy local,” which could increase the average costs of vaccines and decrease the volumes of pooled purchasing. Initiatives in the LAC region to expand local manufacturing capacity must therefore consider potential knock-on effects for cooperative purchasing mechanisms like the PAHO Revolving Fund and either incorporate efforts into mechanisms’ existing policies and financing models or develop better mechanisms and incentives for securing stable demand in the future.
-
Signing long-term contracts and revenue guarantees for routine procurement
Long-term contracts can help ensure stable demand, incentivize investment in manufacturing capacity, and reinforce procurement channels like the PAHO Revolving Fund. However, governments deciding to buy locally or regionally produced vaccines will need to contend with possible trade-offs, such as an increase in the price of vaccines, which may be sold more cheaply elsewhere. Governments need to consider their commitment to paying a premium for local and regional production, including the size of the price increase and how long such a premium can be sustained.
-
Signing at-risk contracts (or advance market commitments) for pandemic-related vaccine manufacturing and procurement
In addition to long-term contracts, governments can sign at-risk contracts or advance market commitments to promise future demand in the event of a health emergency. These contracts enable firms to raise financing to build facilities and maintain workforces to make sure emergency vaccines are available when needed.
Key Elements for Success on the Supply Side
To target their investments in increased manufacturing capacity strategically and successfully, LAC policymakers must also consider these five supply-side factors:
-
Capital investment
Establishing and maintaining the manufacturing facilities and trained workforce needed to produce emergency vaccines requires significant resources. Estimates suggest that $60 billion would be needed up-front to fully expand global production to respond to future pandemic threats. Key sources for this financing include private sector money from future demand contracts and profit earned from competitive markets, in addition to public sector subsidies.
-
Private sector engagement
The private sector will be a crucial source of financing to scale up manufacturing capacity. Multinational firms must also grant access to intellectual property and facilitate technology transfer to enable a quicker and more cost-effective increase in manufacturing in new locations by new producers.
-
Integration with the global system and market
Manufacturers will need to consider their place in the supply chain, identifying sources for critical input materials and defining their intended market as needed. The scale and type of resources required to establish sustainable and effective manufacturing capacity for emergency vaccines will depend on these strategic choices.
-
High-impact products and manufacturing phases
Smart, sustainable investments in manufacturing capacity will reinforce promising, flexible, and adaptable technologies like mRNA vaccines, which can be used against other diseases and reformulated against new variants more easily than other vaccine technologies. Decision makers must also consider their investments within the context of the broader supply chain, coordinating to ensure market diversity and prioritizing cost-effective manufacturing phases that enable better access arrangements when an emergency strikes.
-
Location
Manufacturing capacity will be scaled more efficiently if decision makers base investments off assessments of existing manufacturing capabilities, regulatory capacity, the size of the trained workforce, and the size of the market. The LAC region has six national regulatory authorities of regional reference and a strong foundation of manufacturing capabilities, with two countries in the region producing World Health Organization-prequalified vaccines and five countries already involved – albeit to a limited degree – in the production of COVID-19 vaccines. Decision makers must coordinate with existing initiatives in the region, including those implemented at the country level and/or led by PAHO, to reinforce and harmonize rather than duplicate or undermine efforts.
Conclusion
Decision makers must make proactive and strategic decisions on priority policy goals, acceptable trade-offs, and potential mitigation strategies to scale manufacturing for emergency vaccines effectively and sustainably. Actions taken now will be difficult to undo down the road, so decision makers must ensure that the structural and financial arrangements implemented to produce and buy emergency vaccines promote the best possible outcomes for the population, government purchases, and the market during this pandemic and the next health emergency.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.