Recommended
Much rests with the successful development and introduction of an effective COVID-19 vaccine. It may be our only path towards fully reopening our economies without fear of future outbreaks and associated health and economic impacts. The response of product developers has been swift. Over a hundred vaccine candidates are in the pipeline, and even preliminary data releases from early trials can send the relevant company’s and the overall stock market rallying and cratering, in turn.
Even as science and research progress, governments, CEOs, and the public are all asking how to speed up development, finance late stage development, finance and manage the scale up of manufacturing, and prioritize doses in a supply-constrained environment, with the goal to interrupt global transmission. There has been a flurry of response—including from CGD—to the call for innovative financing, organizational and policy ideas to solve these problems, many drawing on the treasure trove of experience and knowledge earned through solving complex global health challenges.
Faced with the unique challenge of a solution to perhaps the modern world's first truly global health challenge, speed and efficacy are critical. From our perspective, it is crucial to find a solution that leverages the "missing middle"—the private sector and middle-income countries, as well as developed economies. If we are not able to do so, we face the risk that this crucial task becomes an expensive exercise played along traditional donor and recipient lines, where national interests may trump the potential of the best science to advance.
Anyone not steeped in the nuances of vaccine development and global access could be forgiven for not being able to follow and differentiate all the proposals and associated acronyms. At least four new initiatives geared at accelerating accessible vaccine development and manufacture have either been launched or received a lot of media attention over the past month.
Recent vaccine initiatives:
Development of a vaccine is one of three product pillars of the Access to COVID-19 Tools (ACT) Accelerator, a platform through which leading global health organizations—WHO, GAVI, CEPI, the Bill & Melinda Gates Foundation, the Global Fund, Wellcome Trust—together with industry and government partners have committed to coordinate efforts to realize global solutions that include the poorest in low and low-middle income countries (LMICs). While the ultimate vision is that of end-to-end solutions, funding has, so far, been focused on R&D, most recently through a $8 billion EU-driven pledging drive. CEPI and GAVI are co-leads for the vaccine pillar, referred to as COVAX (COVID-19 vaccine access).
The US government has announced an intensive government-supported effort, “Operation Warp Speed,” to advance a portfolio of 14 promising vaccine candidates, including funding for trials and, later, manufacturing scale-up (pre-launch) for a smaller subset of three to five particularly promising candidates. This appears to be an exclusively US-focused effort supporting mostly or entirely US-developed vaccines but with some recent signaling that the US will cooperate with other nations.
In response to the specific challenge of scaling manufacturing capacity to meet what is expected to be unprecedented global demand, the AcceleratingHT group, led by Nobel Prize-winning economist (and CGD affiliate) Michael Kremer, has proposed what they are referring to as an Advanced Market Commitment. While their ideas are evolving over time, i.e. covering the US and/or being more globally focused, at the core is a call for combination of portfolio optimization and significant amounts (up to $70bn for the US alone, twice that for a global effort) upfront, of government push funding to build manufacturing capacity at risk, before the anyone knows which vaccine candidates, if any, will be successful, dramatically to accelerate development and delivery.
In response to the specific challenge of securing sufficient accessible vaccine supply for LMICs, Gavi, the Vaccine Alliance, has proposed a $2 billion donor-funded advanced market commitment (seed amount, likely to increase) for 20 million doses of a COVID-19 vaccine to be used in low- (and possibly middle-) income countries, with priority to health workers. Both the GAVI and the Kremer proposals, are focused on the manufacturing capacity challenge, implicitly assuming that a combination of push (CEPI, BARDA) and private sector investment (in expectation of lucrative high-income markets) will drive the product R&D.
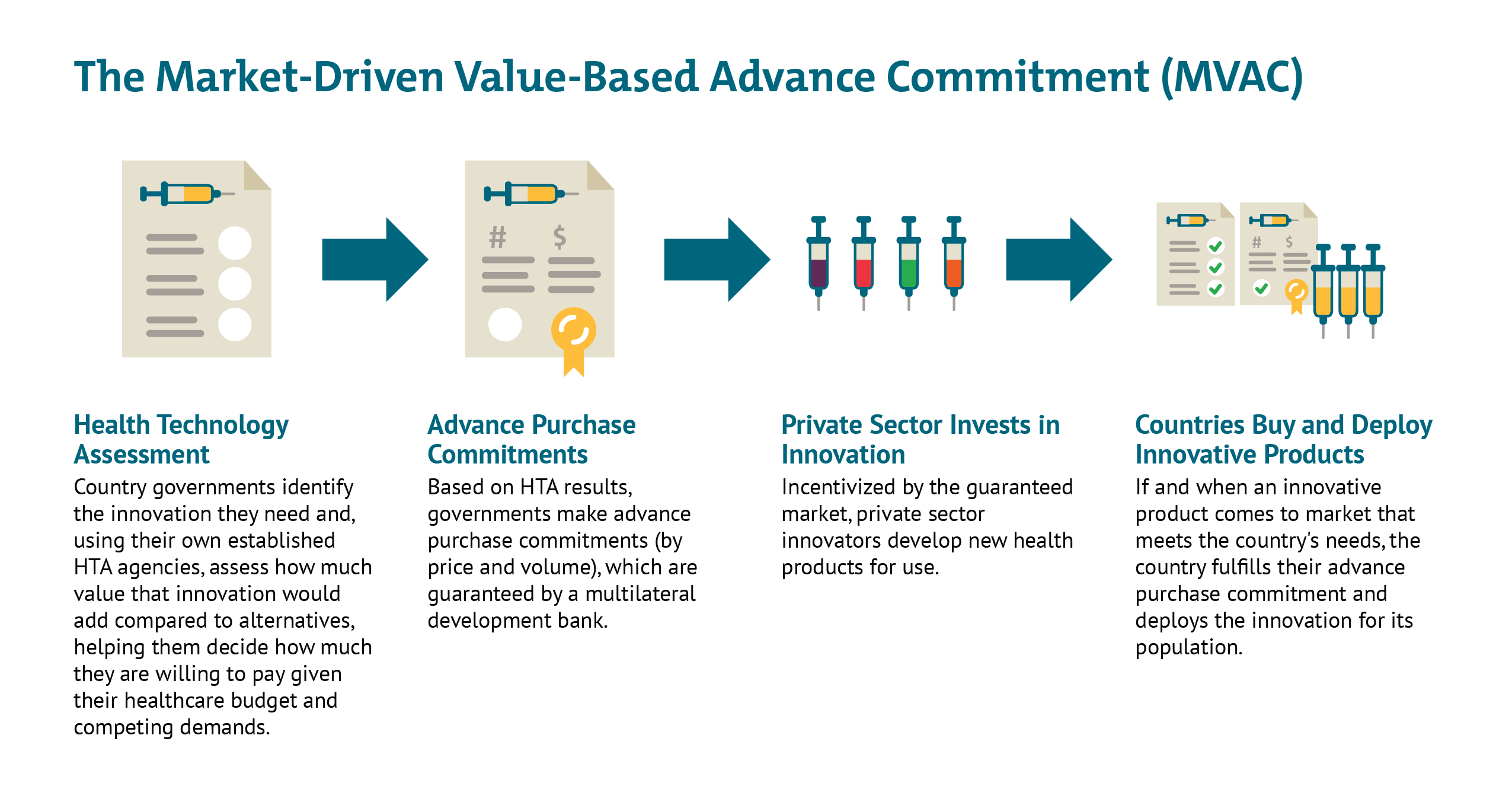
The global health policy team at CGD, with colleagues from the Office of Health Economics and PATH, have developed a concept we refer to as a benefit-based advanced market commitment (BBAMC), that builds on the strengths of the GAVI and Accelerating HT proposals. The BBAMC differs from these proposals by offering a model that is explicitly targeted at middle-income countries, as well as the high-income and low, and builds an advanced commitment based on future expected health care expenditures (on COVID-19 vaccine), not just donor aid. It also pools the individual commitments of high-income countries, bringing clarity on the size of the overall global market and therefore enabling priorities of allocation to be addressed. See these links to our preliminary research, the full proposal, and a recent opinion piece outlining the concept.
Faced with the unique challenge of a solution to perhaps the modern world's first truly global health challenge, speed and efficacy are critical. From our perspective, it is crucial to find a solution that leverages the "missing middle"—the private sector and middle-income countries, as well as developed economies. If we are not able to do so, we face the risk that this crucial task becomes an expensive exercise played along traditional donor and recipient lines, where national interests may trump the potential of the best science to advance. Building on the strength of the proposals from AccelerateHT, GAVI, the ACT Accelerator and others, our benefit-based AMC allows push funding to be more heavily concentrated to support early science and mitigating some of the manufacturing fixed cost risks, where it is most efficient, and creates a value-based market that will allow pull funding to drive the best candidates forward through late stage development and manufacturing. For more information see our slide deck, and annex.
We are all building the boat as we sail it. At a time when the modern world faces what is perhaps our first truly global health challenge, we are learning that an inclusive environment that fosters open debate and collaboration between public and private players in wealthier and emerging markets is not an optional extra.See the first page of our slide deck below, and click here for the full presentation.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.
Image credit for social media/web: Leigh Prather via Adobe Stock